Introduction
The availability of fluorescent dyes or molecules, such as organic fluorophores and naturally occurring luminescent proteins (e.g., green, yellow, and red fluorescent proteins), have advanced our understanding of molecular and cell biology through specific labeling of targets expressed in cells and tissues. This study advancement has been accompanied by developments in (confocal and super-resolution) fluorescence microscopy, (single-molecule) fluorescence spectroscopy, and flow cytometry. When fluorescent molecules are excited with an appropriate energy light source, there is the spontaneous emission of lower Energy Photon in a process known as fluorescence. These emitted photons possess broad emission wavelength characteristics (see representative simplified Jablonski diagram in Fig. 1a, 1c).
As a very different approach, under certain circumstances, this spontaneous emission can be overcome by stimulated emission. Stimulated emission (Fig. 1b), coined for the first time by Albert Einstein in 1917, is an optical phenomenon in which an incoming photon of a specific wavelength interacts with the electron at the excited state, followed by relaxation to a lower ground energy level resulting in a new photon with identical phase, frequency, polarization, and direction of propagation as the photon source. This process is widely known as the foundation of light amplification in laser technology (i.e., laser stands for light amplification by stimulated emission of radiation). Depending on the type of gain medium, lasers are classified into different types (i.e., gas, solid-state, semiconductor, and organic lasers). In an organic laser setup, amplified stimulated emission can be obtained by optically pumping an active/gain medium (fluorescent dye), which is placed inside a well-defined optical cavity, typically a sandwich of highly reflective mirrors providing coherent optical feedback (Fig. 1d).
Read more :
When the total gain inside the cavity is larger than the losses, the system reaches the threshold for lasing action to occur. The resulting laser emission is coherent and very narrow in terms of the spectrum in comparison to the fluorescence signal (Fig. 1c). A bio laser is a new class of organic lasers, defined as the integration of biomolecules or biomaterials in the laser system (Fig. 2). As with some (organic) fluorescent chromophores, certain materials (typically fluorescent biomolecules) can be used as the gain medium. Bio lasers have been achieved by placing green fluorescent proteins (GFP) or vitamins inside the optical cavity, e.g., Fabry-Pérot or distributed feedback (see Fig. 2a).
Biomaterials and biopolymers such as plant starch, egg white, polylactic acid, and poly(lactic-co-glycolic acid), which form natural spherical or ellipsoidal micro granules shapes, can also act as high-quality whispering-gallery-mode (WGM) resonators to provide sufficient optical gain for lasing. Another possible scenario is to use biomaterials, which are typically created as nano- and micro-structures serving as scatterers that can effectively provide optical feedback (i.e., in the case of random bio lasers, Fig. 2b). Non-fluorescent biomaterials can be further employed as interference/disturbance medium in the lasing systems (inside the cavity). In this regard, the alteration in lasing properties (i.e., increase of the optical losses due to unwanted scattering or refractive index difference) is expected, and it can reveal the optical properties of biomaterials. Organic micro/nanolasers have been significantly advanced over the last decade, especially related to the continuous improvement in controlling their lasing output. Different designs and controls of microcavities and organic optical gain media have been developed to build high-performance micro/nanolasers (i.e., wavelength-tunable lasers, multi-wavelength lasers, single-mode lasers, mode controllable lasers, and directional lasers). Among them, dual-wavelength single-mode lasing based on a mutual model selection mechanism was demonstrated in axially coupled organic heterogeneous nanowire resonators, in which each individual nanowire functions as both laser source and mode filter for the other nanowire. These nanowire resonators provided multiple nanoscale output ports to produce coherent signals with various colors increasing the integration level of such photonic devices. Besides, broadband tunable single-mode microlasers based on photoisomerization activated intramolecular charge-transfer process is coupled polymer microdisk cavities were also reported.
In that review, the single-mode lasing could be reversibly tuned between ∼699 nm and ∼726 nm by alternating the UV and Vis irradiation. Host-guest composite organic microlasers have been further researched to reduce lasing thresholds, improve laser lifetime, and extend the tuning range. In such a configuration, the host materials do not only improve the lasing performances but also provide new functionalities for the organic microlasers, which it is expected can be more beneficial when used in real practical applications (e.g., toxic gas detection, biological sensing, and color laser display). For controlling the output of organic micro/nanolasers, current techniques are commonly based on external stimuli (i.e., light, force, heat, and vapor). However, in the case of practical applications (e.g., hybrid optoelectronic integration and interconnects), electrically controlled organic micro/nanolasers are more desirable.
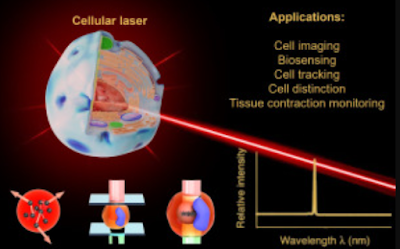
Biological cells have recently emerged in the lasing system and have successfully been employed to generate laser action, which from this point onwards will be referred to as cellular lasers. In particular, cells or bacteria can be engineered to express certain fluorescent proteins. Unlike in a conventional fluorescence imaging setup, in which fluorescence signals are detected, once those entities have been placed inside an optical cavity, generation of stimulated emission upon sufficient optical pumping is very likely to occur (Fig. 2c). Cells exhibit different auto-fluorescence signals arising from endogenous molecules such as mitochondrial nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD), which can be used as an intrinsic photon source for light amplification.
Moreover, the advances in the fields of cell staining and immunolabeling have allowed cells, including their cellular organelles (e.g., nuclei, lysosomes, endosomes, golgi complex, mitochondria, and cytoplasm) and cellular architectures (F-actins, microtubules, and intermediate filaments) to be specifically tagged with certain organic fluorophores or fluorescent-labeled antibodies. This opens the possibility to generate lasers from different sites within cells and more importantly, allows the production of cellular laser activity with more than single-wavelength laser radiation (i.e., multiplexing) upon multiple labeling. Lastly, the possibility to introduce fluorescent/luminescent particles (e.g., quantum dots, carbon dots, semiconductor nanowires, and fluorescently-labeled nanoparticles as gain media or probes into the cells can be also beneficial in developing cellular lasers.
Apart from placing a single cell or bacterium inside an optical cavity (or termed an external cavity), resonators can be also miniaturized and introduced inside the cell (i.e., internal cavity, Fig. 2d). The nature of the cells that tend to internalize objects of certain sizes (typically less than 1–2 µm) using a process called endocytosis will be important knowledge to deliver the cavity into the cytoplasmic region of the cells. In case the resonator is too large to be internalized naturally, transfection technology utilized for delivering DNA/RNA into the cells can be also opted. Once the cavity is present inside the cells, intracellular fluorescence coming from fluorescent proteins or organic fluorophores can be optically coupled to the cavity, and subsequently amplified.
In this review, all possible scenarios to generate cellular lasers are discussed. The potential applications of cellular lasers for next-generation cell imaging and cytometry as well as for cell analysis and distinction study are emphasized, while key remaining challenges in engineering the cellular lasers are highlighted.
The possibility to produce laser action involving biomaterials, in particular (single) biological cells, has fostered the development of cellular lasers as a novel approach in biophotonics. In this respect, cells that are engineered to carry gain medium (e.g., fluorescent dyes or proteins) are placed inside an optical cavity (i.e., typically a sandwich of highly reflective mirrors), allowing the generation of stimulated emission upon sufficient optical pumping. In another scenario, micron-sized optical resonators supporting whispering-gallery-mode (WGM) or semiconductor-based laser probes can be internalized by the cells and support light amplification. This review summarizes the recent advances in the fields of bio lasers and cellular lasers, and most importantly, highlights their potential applications in the fields of in vitro and in vivo cell imaging and analysis. They include biosensing (e.g., in vitro detection of sodium chloride (NaCl) concentration), cancer cell imaging, laser-emission-based microscope, cell tracking, cell distinction study, and tissue contraction monitoring in zebrafish.
Lastly, several fundamental issues in developing cellular lasers including laser probe fabrication, the biocompatibility of the system, and alteration of the local refractive index of optical cavities due to protein absorption or probe aggregation are described. Cellular lasers are foreseen as a promising tool to study numerous biological and biophysical phenomena.
Bio lasers are a generation of lasers involving biological materials. Biomaterials, including single cells, can be engineered to incorporate laser probes or fluorescent proteins, or fluorophores, and the resulting light emission can be coupled to an optical resonator, allowing the generation of cellular laser emission upon optical pumping. Unlike fluorescence, this stimulated emission is very sensitive and is capable of detecting small alterations in the optical property of the cells and their environment.
In this review, recent development and applications of cellular lasers in the fields of in vitro and in vivo cell imaging, cell tracking, biosensing, and cell/tissue analysis are highlighted. Several challenges in developing cellular lasers including probe fabrication and biocompatibility as well as alteration of the cellular environment are explained.
Applications of cellular lasers
Promising applications of cellular lasers are in the fields of cell imaging, cell classification, biomechanical analysis, and biosensing. The generation of cellular lasers in general can give major advantages, such as improving the resolution and sensitivity of microscopic cell imaging. The intensity of laser emission possesses a multifold increase in the brightness compared to fluorescence emission, allowing the production of higher contrast images and overcoming the depth penetration issue during imaging of thick biological samples.
Generation of cellular lasers
One of the rising topics in the field of bio lasers nowadays is devoted to the involvement of biological organisms (e.g., single cells) in the lasing system. The general idea behind this is that single cells expressing fluorescent protein when placed inside an optical cavity, upon sufficient optical pumping, allow amplified stimulated emission to be generated.
Furthermore, Yun et al. and Gather et al. succeeded to extend the idea of cellular lasers by introducing the concept of lasers with both the gain medium and cavity being present inside cells
Recently, the integration and generation of inorganic intracellular lasers were demonstrated to have a WGM resonator with a size of < 1 µm. From that work, an intracellular laser with volumes 1000-fold smaller than the eukaryotic nucleus (Vlaser < 0.1 µm3) and lasing thresholds 500-fold below the pulse energies typically used in two-photon microscopy (Eth ≈ 0.13 pJ), as well as excellent spectral stability (<50 pm wavelength shift), was successfully achieved employing aluminum gallium phosphide (AlGaP) multi-quantum well nanodisks.
In addition to CdS nanowires and InGaAsP microdisks, gallium nitride (GaN) micro rods, which have normally been used by researchers as a building block for developing light-emitting diodes (LEDs) and field-effect transistors (FETs).
This review has highlighted the advancement of laser emission generation involving biological materials, including single cells. Different approaches and examples of cellular lasers, both with and without conventional optical cavities (e.g., Fabry–Pérot and whispering-gallery-mode (WGM)), have been described. In addition, unlike fluorescence which is less sensitive to the change in environments, the resulting laser possesses different properties (e.g., wavelength shifts) depending on the types and biological states of the cells, therefore opening a new possibility to be used for cell imaging and analysis. Moreover, several in vitro and in vivo biosensing applications (e.g., detection of salt concentration, cancer cell distinction (cytometry), and monitoring of cell mechanical properties) have been demonstrated using the developed cellular lasing technique.
Few challenges in designing the cellular laser experiment and laser probe including biocompatibility of the system and alteration of the local refractive index of optical cavities due to protein absorption or probe aggregation should be considered. The field of cellular laser has not been mature yet. However, this promising technology is expected to be further developed and employed to study many important biological and biophysical phenomena, in which the laser performance can be improved by its combination with nanotechnology (e.g., plasmonic enhancement by metallic nanoparticles), microfluidic, and deep learning approaches.
Future challenges and outlooks
Several aspects need to be considered while designing a cellular laser and its experiments, which include the following points:
While performing a cell seeding inside the cavity, the cell might not attach/adhere properly to the cavity wall due to its non-biocompatibility feature. This will lead to a change in cellular properties and in an extreme case can lead to cell death. Pre-functionalization of the cavity surface with a very thin layer of proteins (e.g., collagen or fibronectin) or biocompatible polymers (e.g., poly-l-lysine) can be employed as a strategy to improve cell adhesion.
The formation of abundant protein corona on the internal cavity can alter the refractive index of the cavity surface, therefore changing the lasing properties. The walls of the external cavity can technically adsorb abundant protein by means of electrostatic/van der Waals interaction. Polystyrene particles used as a WGM cavity have been demonstrated to adsorb certain types of proteins upon introduction to cell media. The use of serum-free medium or buffer (e.g., phosphate-buffered saline) is therefore suggested.
WGM resonators (e.g., polystyrene particles) and laser probes (e.g., fluorescent particles or semiconductor nanowires) can aggregate in cell media and inside the cell, which is not desired. Proper (chemical) surface functionalization is needed to make the probe colloidally stable.
Internalized WGM spheres or nanowires are likely to end up in the lysosomes, and digestive organelles of cells with very high acidity. In the long term, it can result in the destruction of the probes.
Biocompatibilities of the cavity, laser probe, and gain medium need to be considered while designing living cellular lasers. Therefore, the choice of materials should be planned accordingly. As an example, a very high concentration of fluorescent dyes has been shown to be toxic to cells.
Most cells naturally do not possess spherical geometry as they are adherent on the surface of culture plates. To produce spherical cells, proteolytic enzyme solutions (e.g., trypsin or accutase) can be beforehand used to detach the cell from the surface. Detached cells are normally round in shape.
Multiple labeling with different fluorophores targeting different organelles is possible, therefore providing an opportunity to produce multiple laser emissions from a single cell.
Fabrication of monodisperse microsphere bio lasers plays an important role to result in highly reproducible and stable WGM lasing performance. A microfluidic-based fabrication technique can be employed to generate monodisperse dye-doped protein microsphere bio lasers with a tunable size range of 50 – 150 µm. Meanwhile, obtaining an optofluidic ring resonator (OFRR) with the same size and wall thickness, and industrial fiber draw tower can be used to overcome reproducibility issues raised in lab-based low-cost fabrication techniques using CO2 laser pulling machines.
The bio-extracted materials utilized for bio lasers normally require complicated extraction and synthesis processes, which consequently suffer from high costs. Using a simple dehydration method or its modified process (microglassification™), low-cost microspheres made of various natural materials (e.g., goose egg white, bovine serum albumin (BSA), and chicken albumen) can be reproducibly created in different sizes.
Cavity with a high Q-factor and low threshold is highly desirable to minimize the biological damage of external pumping light and concentrated fluorophores to living cells and tissues. This will enhance the laser lifetime. It should be noted that efficient lasing emission only occurs above the lasing threshold.
The penetration of the commonly used lasers for excitation (e.g., UV – visible light (190 – 700 nm) and NIR‐I light (700 – 950 nm)) is limited to a depth of < 10 mm into the subcutaneous tissues. Further design and development of gain materials, which can be excited in the NIR‐II light region (1000 – 1700 nm), will contribute to deep tissue applications. The other feasible solutions are combining bio lasers with biocompatible optical waveguides that can transport light into deep tissues and using a two-photon imaging technique that provides less optical damage to cells and tissues.(Indrawan Vpp)
READ MORE ABOUT SCHOLARSHIPS:
New Information Scholarship Program 2022 at the University of Cambridge in UK
The Graduate Excellence Fellowships in University of Geneva 2022
Singapore Scholarship 2022 - SINGA